What Happens When HCOOCH CH OH React? A Deep Dive Into This Unique Organic Chemistry Scenario
In the world of organic chemistry, understanding the behavior of various compounds when mixed under different conditions is essential not only for academic purposes but also for practical applications in industries ranging from pharmaceuticals to green energy. One such intriguing chemical mix involves the combination: HCOOCH CH OH.
If this sequence of molecules has you curious—whether you’re a student preparing for an exam, a researcher exploring synthetic pathways, or simply a chemistry enthusiast—this post will serve as a comprehensive guide. We’ll break down what these compounds are, how they interact, and the chemical and industrial relevance of this reaction, using real-world contexts and the latest insights available in 2025.
Understanding the Reactants: A Quick Primer
HCOOCH – Methyl Formate
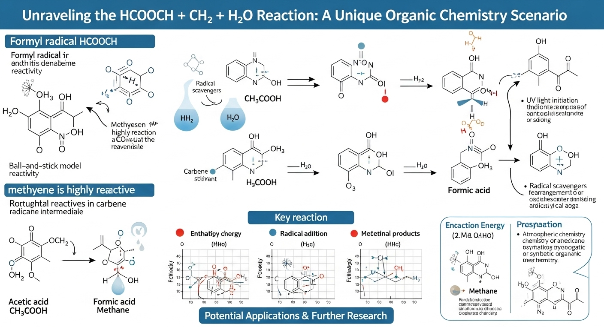
HCOOCH, more commonly known as methyl formate, is an ester derived from formic acid and methanol. It has the structural formula HCOOCH₃. It appears as a colorless, volatile liquid with a pleasant smell, often used as a solvent or intermediate in organic synthesis.
In atmospheric chemistry and astrochemistry, methyl formate has been found in interstellar space, indicating its importance in prebiotic chemical evolution. But more practically, it’s a key ingredient in making formamide, and even in low-global-warming-potential blowing agents for foam insulation.
CH₂ – Methylene Group (or Carbene?)
This notation can get ambiguous without context. CH₂ can either refer to:
-
Methylene group (-CH₂-), as a bridge between two atoms.
-
Carbene (CH₂), a highly reactive and short-lived species with a divalent carbon.
In reaction contexts, the carbene form is more likely, especially when considering reactive intermediates in organic transformations. Carbenes are unstable and extremely reactive, often acting as intermediates in complex organic reactions like cyclopropanation, insertions, or ylide formation.
H₂O – Water
No mystery here—water is the universal solvent, and its role in organic chemistry varies from acting as a reaction medium to being a reactant itself. In our context, its presence could signal hydrolysis, proton transfer, or a medium for catalysis.
So, What Happens When HCOOCH CH OH React?
To analyze this properly, we need to hypothesize the possible reaction mechanisms and products. Here’s a realistic take based on established organic chemistry:
1. Hydrolysis of Methyl Formate in Water
In the presence of water, methyl formate (HCOOCH₃) can undergo hydrolysis—a common ester reaction—especially in acidic or basic conditions. The result:
This reaction yields formic acid (HCOOH) and methanol (CH₃OH). Now, we have new reactants in the system, and this opens the door for interactions with the CH₂ species.
2. CH₂ as Carbene: Insertion Reactions
If CH₂ is a carbene, it’s a game-changer. Carbenes are known for their high reactivity and can insert into single bonds, including C-H or O-H bonds. So if CH₂ is generated (for instance, from diazomethane or thermolysis), it could react as follows:
-
With formic acid (HCOOH), possibly leading to:
(Hypothetical product: glycolic acid, depending on mechanism)
-
With methanol, we might get:
Though this is less likely without catalysis.
These hypothetical products depend heavily on reaction conditions—temperature, pressure, catalyst, and solvent all matter.
Real-World Applications and Relevance
Industrial Chemistry
The hydrolysis of methyl formate is an essential process in formic acid production, a compound widely used in leather tanning, rubber manufacturing, and agriculture (as a silage preservative). Understanding side reactions involving CH₂ could help chemists optimize yields, reduce unwanted by-products, or even explore green synthesis pathways.
Organic Synthesis
Carbenes are increasingly relevant in transition-metal catalyzed organic synthesis. If CH₂ is generated in situ, it could pave the way for cyclopropane ring formation or carbon-carbon bond insertions—a technique used in synthesizing pharmaceutical precursors.
Academic Research and Astrochemistry
The combination of these molecules has been studied in astrochemical models. For example, both CH₂ and methyl formate have been detected in star-forming regions. Research in 2025 continues to explore how these molecules interact under cosmic conditions to potentially form biologically relevant compounds like amino acids or sugars.
Reaction Summary Table
| Reactant | Role in Reaction | Possible Product | Notes |
|---|---|---|---|
| HCOOCH₃ | Ester, hydrolyzes in water | HCOOH (formic acid), CH₃OH | Catalyzed by acid/base |
| CH₂ | Carbene (reactive) | Inserts into H-X bonds, forms C-C or C-O bonds | Requires careful generation |
| H₂O | Solvent, hydrolyzing agent | Facilitates ester hydrolysis | Key medium |
Actionable Takeaways
-
Understand the context of CH₂ – Whether it’s a methylene bridge or carbene drastically changes reaction outcomes.
-
Watch the water – It doesn’t just “dilute” things. Water actively participates in hydrolysis and proton transfer.
-
Ester chemistry is everywhere – Even a simple ester like methyl formate has wide industrial use.
-
Carbenes are synthetic gold – But only if you can control them.
Practical Example: Lab-Scale Ester Hydrolysis and Carbene Reaction
Let’s say you’re in an undergrad lab, and you mix methyl formate and water under heat and mild acid. You get formic acid and methanol, no surprise.
But now imagine you slowly add diazomethane to the system to generate CH₂ carbenes. With careful control, you might observe formation of additional carbon-based products—an example of how these simple compounds interact in controlled organic reactions.
Conclusion: More Than Just Letters on a Page
What began as a cryptic chemical expression—HCOOCH CH OH—turns out to be a window into the deeper mechanics of organic chemistry. These molecules, though small, demonstrate some of the most powerful concepts: reactivity, transformation, and synthesis. Whether you’re thinking academically or industrially, understanding this combination means appreciating how small steps drive major innovations in science and engineering.
FAQs About HCOOCH CH OH
Q1: Is CH₂ always a carbene in such reactions?
Not necessarily. It can also appear as a part of larger molecules. But in standalone reactions or those involving diazo compounds, it likely refers to a reactive carbene.
Q2: Can I safely perform reactions involving CH₂ in a high school lab?
Definitely not. Generating CH₂ as a free carbene often involves hazardous reagents like diazomethane, which are extremely toxic and explosive. These are best left to professional research environments.
Q3: Why is water important in this reaction?
Water doesn’t just dissolve substances—it actively participates. In this case, it helps break down methyl formate via hydrolysis, yielding formic acid and methanol.
Q4: What’s the real-world use of methyl formate?
It’s widely used in the production of formic acid, solvents, and blowing agents for foam. It’s also being explored in green chemistry for its low toxicity and environmental impact.
Q5: Could this combination occur naturally, like in space?
Yes! Methyl formate and reactive carbon species like CH₂ have been detected in interstellar clouds. These reactions are helping researchers understand how complex organic molecules may have formed before life began.
Q6: What’s a simple way to remember ester hydrolysis?
Think of it like this: an ester plus water gives you an acid and an alcohol. It’s like breaking a sandwich into its bread and filling components.
If you’re a student or chemist and found this breakdown of HCOOCH + CH₂ + H₂O useful, share this with your peers or bookmark it for exam prep. Chemistry is complex, but with the right perspective, even the toughest-looking formulas become meaningful.

![Swimsuit Edition [ABBB] - 1.20 21 Swimsuit Edition - Chapter](https://newzio.pro/wp-content/uploads/2025/07/AQN_u332c2tRFy_6zmICQjgeTpRyLz0oTYkJoZAwlw8OJZdfnCvwKawGC-HWhremQ33DTul7DJAR5q1zgH0Y2X59zraz-AleA5Ddx6HKSI-KGUJiB4FwDCM2e_UfRPAgFt7sa2KelpwXkczEnPTwbXyk1RSP.jpeg)

Leave a Reply